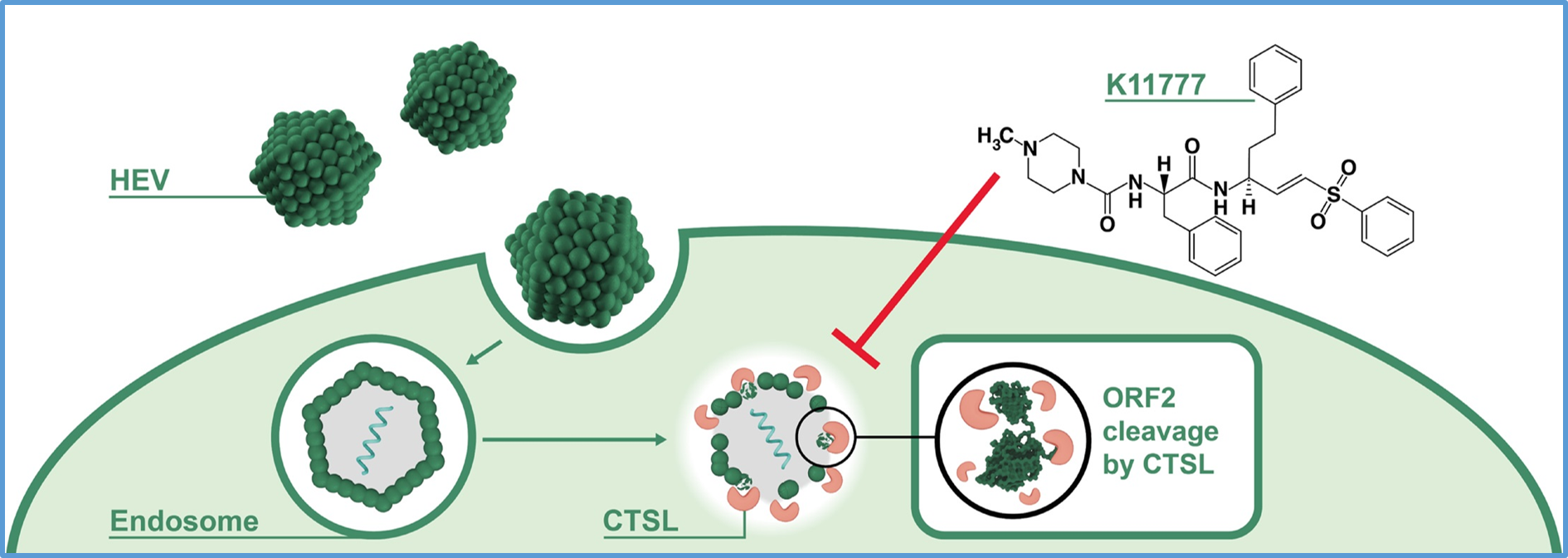
The hepatitis E virus (HEV) is the main cause of acute viral hepatitis. Approximately 70,000 people die from the disease every year, yet therapy options remain limited. In the pursuit of effective antiviral therapies, targeting viral entry holds promise and has proven effective for other viruses. Since cellular proteases have emerged as host factors required for productive cell entry by many viruses, a recent study led by Prof. Eike Steinmann from Ruhr University Bochum in Germany investigated the functional requirement and therapeutic potentials of cellular proteases during HEV infection.
The researchers found that blocking lysosomal cathepsins (CTS) with small molecule inhibitors, impedes HEV infection without affecting replication. Most importantly, the pan-cathepsin inhibitor K11777 robustly suppressed HEV infections in hepatoma cells, HepaRG and primary human hepatocytes. Furthermore, through time-of-addition and RNAscope experiments, they confirmed that HEV entry is blocked by the inhibition of cathepsins. Cathepsin L (CTSL) knockout cells were less permissive to HEV, suggesting that CTSL is critical for HEV infection. Finally, they observed the cleavage of the glycosylated ORF2 protein and virus particles by recombinant CTSL.
This study highlights the pivotal role of lysosomal cathepsins, especially CTSL, in the HEV entry process. However, further studies will be essential to define putative interactions between the HEV virion and CTSs. In addition, the profound anti-HEV efficacy of the pan-cathepsin inhibitor, K11777, further underscores its potential as a promising therapeutic candidate.
Read the full article online published in Hepatology in May 2024: DOI: 10.1097/HEP.0000000000000912