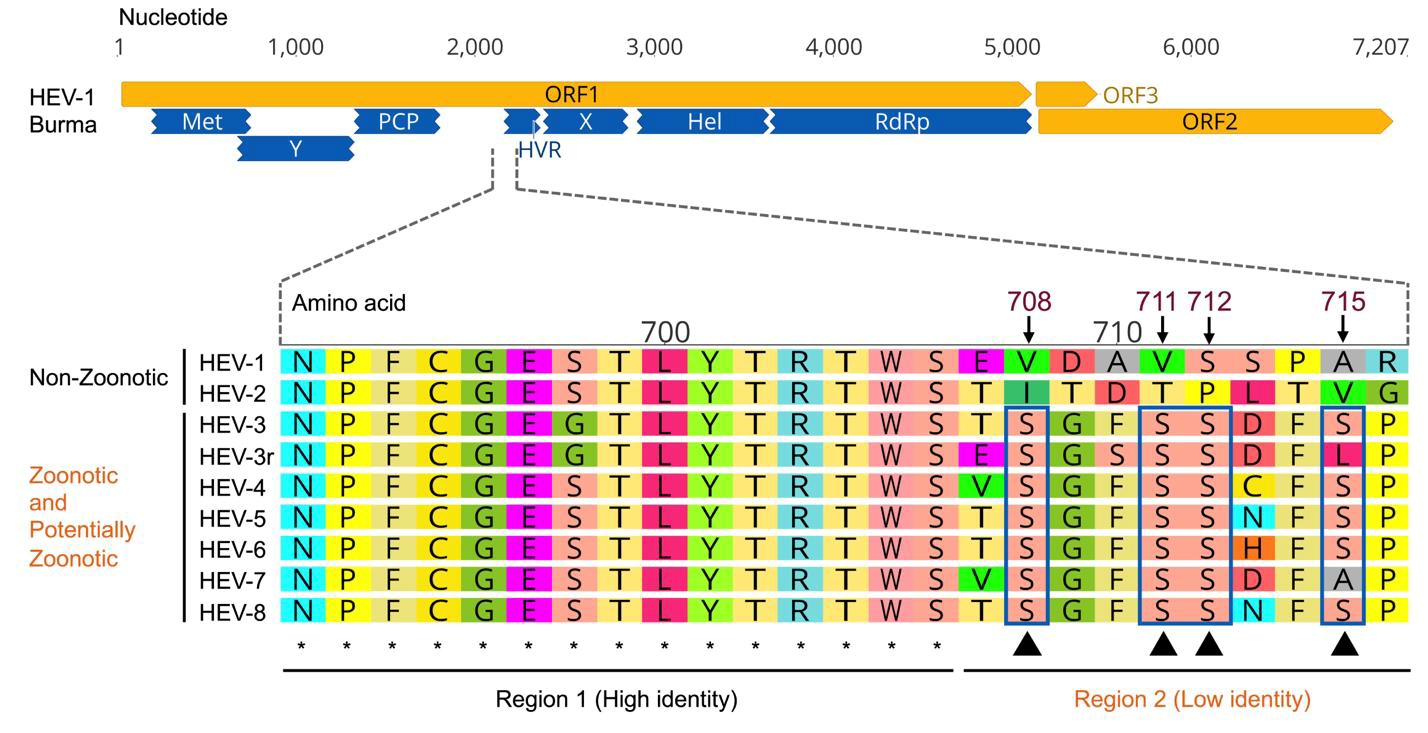
Hepatitis E virus (HEV) is distinct from other hepatotropic viruses in that it is zoonotic and can infect across species barriers. HEV-1 and HEV-2 exclusively infect humans, whereas HEV-3 and HEV-4 are zoonotic infecting human and several other animal species. The viral and/or host factors responsible for HEV cross-species infection remain elusive. The hypervariable region (HVR) in HEV is extremely heterogenetic and has been implicated in HEV adaptation.
A recent study, led by Dr. Bo Wang and Prof. Xiang-Jin (“X.J.”) Meng from the Virginia Polytechnic Institute and State University, USA, investigated the potential role of serine phosphorylation in the HVR in HEV replication. They first analyzed HVR sequences across different genotypes of HEV and identified a unique region at the N-terminus of the HVR, which is variable in the human-exclusive HEV genotypes but relatively conserved in zoonotic HEV genotypes. They further identified four potential phosphorylation sites that are highly conserved in zoonotic HEV-3 and HEV-4 genomes but are absent in human-exclusive HEV-1 strains. To explore the functional significance of these putative phosphorylation sites, they introduced mutations into the HEV-3 infectious clone and indicator replicon, replacing each Serine residue individually with alanine or aspartic acid, and assessed the impact of these substitutions on HEV-3 replication. They found that the phospho-blatant S711A mutant significantly reduced HEV-3 replication, whereas the phospho-mimetic S711D mutant modestly reduced virus replication. However, mutations in the other three Serine residues did not significantly affect HEV-3 replication. Furthermore, they showed that Ser711 phosphorylation did not appear to alter host cell tropism of zoonotic HEV-3.
The findings from this study showed that potential phosphorylation of the Ser711 residue significantly affects HEV-3 replication in vitro, thus providing new insights into the potential mechanisms of zoonotic HEV transmission.
This study was published in mBio (October 2024) Pages e0263524. Read the full article