Globally, pegylated interferon-α and ribavirin are the only recommended antiviral agents for treating chronic hepatitis E patients. However, though ribavirin induces a sustained virologic response, severe side effects and emergence of drug-resistant HEV mutants in a proportion of patients restricts its use. Therefore, designing of direct-acting or host-targeting anti-HEV agents and identifying the alternative treatment modules are highly needed.
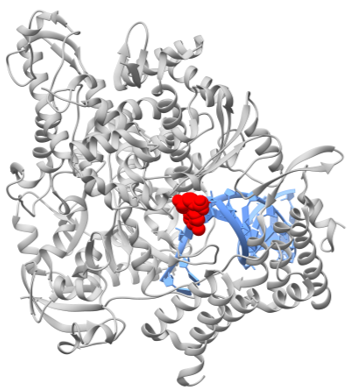
The HEV genomic RNA encodes its largest gene (ORF1) into a nonstructural polyprotein (pORF1) defined into seven domains including RNA-dependent RNA polymerase (RdRp). However, whether pORF1 is a multi-functional polyprotein or gets processed into individually-active small proteins, still remains contested. Because RdRp is indispensable for viral RNA replication, it is considered as potential anti-HEV drug target.
A collaborative work of Prof. Mohammad K Parvez (King Saud University, Riyadh, Saudi Arabia) and Prof. Deepak Sehgal (Shiv Nadar University, Greater Noida, India) has explored potential of FDA-approved viral RdRp inhibitors, notably, favipiravir, sofosbuvir, remdesivir, filibuvir, and tegobuvir against HEV RdRp. To screen the drug candidates, both in vitro (HEV-RdRp expression and enzyme inhibition etc.) and in cellulo (HEV replicon-cell culture, RNA quantification and Immunofluorescence etc.) approaches were adopted. Of these, while monotherapy with favipiravir and sofosbuvir markedly inhibited the RdRp activity, their combination therapy showed a reduction in viral RNA copy numbers by approximately 90%. Therefore, favipiravir and sofosbuvir combination has been proposed as a promising anti-HEV regimen warranting further molecular and pre-clinical validations.
Read the full article recently published in ACS Omega 2023 8, 41570 (https://doi.org/10.1021/acsomega.3c05637).