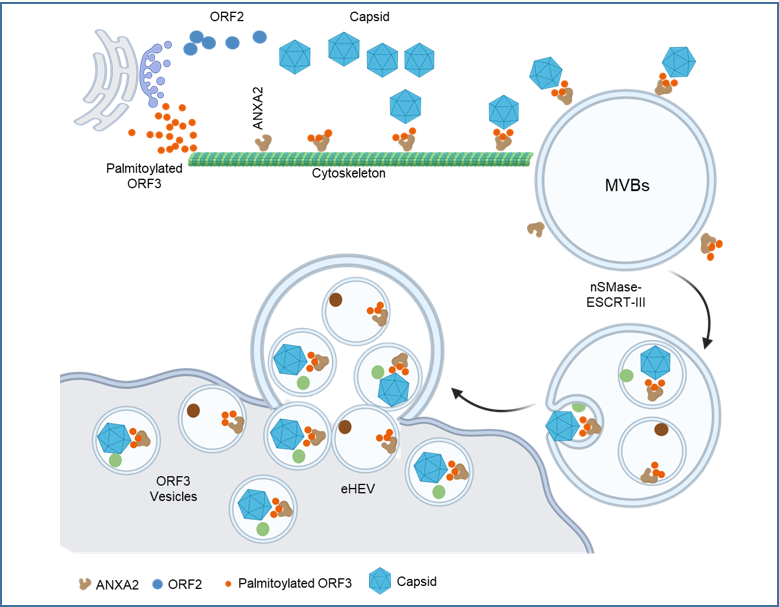
Historically classified as nonenveloped, hepatitis E virus (HEV), an important zoonotic pathogen, has recently been found to exit infected cells as quasi-enveloped HEV (eHEV). These quasi-enveloped virions circulate in the blood and exhibit resistance to neutralizing antibodies, enabling the stealthy spread of infection. Despite strong evidence for the essential role of the HEV-encoded ORF3 protein in eHEV formation, the underlying mechanisms have remained unclear.
A research team led by Dr. Xin Yin from the Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, in collaboration with Dr. Lin Wang’s group from the School of Basic Medical Sciences, Peking University Health Science Center, has uncovered the self-secretory nature of ORF3 vesicles and their critical role in eHEV egress. The study reveals that palmitoylation-dependent interaction with the host protein Annexin II (ANXA2) facilitates ORF3 sorting into vesicles and quasi-enveloped virions. These findings fill a key knowledge gap in the assembly and release of quasi-enveloped virions mediated by ORF3 and open pathways for developing therapeutic strategies to control HEV infection.
Initially identified in the early 1980s, HEV was long considered a nonenveloped virus. However, recent research shows that HEV exists in a quasi-enveloped form (eHEV) in the blood of infected patients and in culture fluids of infected cells. Unlike the nonenveloped virions found in feces, eHEV virions contain additional components, including lipid membranes derived from internal cellular membranes and the ORF3 protein. This protein plays a critical role in eHEV formation and secretion, yet its specific function in these processes was previously unclear.
To investigate the role of ORF3 in HEV egress, the research team purified eHEV particles and ORF3-containing vesicles unassociated with viral particles, examining the characteristics of membrane-associated ORF3. Their findings include:
- Self-Secretion via the Exosomal Pathway: HEV ORF3 is secreted from cells through the exosomal pathway, with an inherent capacity to carry other proteins in vesicles. This suggests that quasi-enveloped virions may form during the self-secretion of ORF3.
- Palmitoylation Sites on ORF3: Key palmitoylation sites (C18 and C21) were identified. Mutations in these sites resulted in a 1.5-log reduction in eHEV release without affecting intracellular viral replication.
- Interaction with ANXA2: Palmitoylation is crucial for ORF3 interaction with the host protein ANXA2. ANXA2 knockdown significantly reduced the efficiency of ORF3 secretion and eHEV release.
- In Vivo Confirmation: Using a Mongolian gerbil model, the study demonstrated that ORF3 palmitoylation is necessary for the secretion of infectious particles in vivo. Moreover, ANXA2 inhibitors reduced fecal viral loads during early infection, suggesting potential therapeutic applications.
These findings highlight the critical role of ORF3 and its interaction with ANXA2 in the biogenesis and secretion of quasi-enveloped HEV. The results not only advance our understanding of HEV pathogenesis but also point to potential therapeutic strategies, such as targeting ORF3 palmitoylation or ANXA2 interactions.
Future research will explore the function of secreted ORF3 vesicles, particularly their role in enhancing the efficiency of extrahepatic infection by eHEV. These studies could further elucidate the molecular mechanisms of HEV pathogenesis and broaden the scope of potential interventions.
Read the full article (Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2418751122): DOI: 10.1073/pnas.2418751122