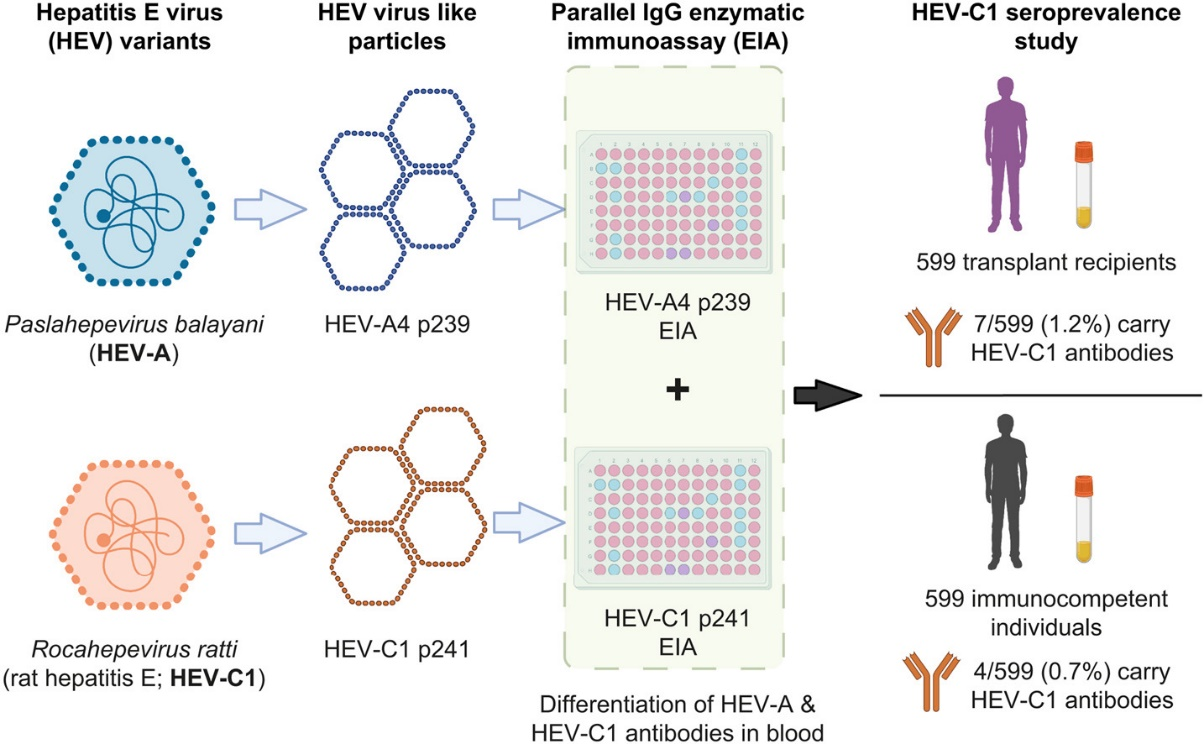
Human cases of Rocahepevirus ratti (HEV-C1) infection are increasingly reported worldwide. Serological tests for HEV-C1 infection are necessary for measuring the clinical burden of HEV-C1 on communities. Currently available commercial hepatitis E serology kits are not very sensitive for HEV-C1 infection and are unable to differentiate between Paslahepevirus balayani genoype 4 (HEV-A4) and Rocahepevirus ratti genotype 1 (HEV-C1) positive patient sera.
In a recent study, the team of Dr. Siddharth Sridhar from the University of Hong Kong developed a parallel IgG enzymatic assay (EIA) system using two virus-like particle peptides (HEV-A4 p239 and HEV-C1 p241). The peptide coating concentrations and serum dilution were optimized by the checkerboard method. Using a panel of confirmed HEV-C1 and HEV-A4 patient sera, cut-offs for individual EIAs and Optical density(OD) HEV-A4 p239/ODHEV-C1 p241 (‘A/C ratio’) were established by Receiver operator characteristic (ROC) curves. Finally, they applied the parallel IgG EIA system to measure HEV-C1 IgG seroprevalence of solid organ transplant (SOT) recipients and immunocompetent patient cohorts. HEV-C1 IgG seropositivity tended to be higher among SOT recipients than individuals who were immunocompetent. The overall measured HEV-C1 seroprevalence in the Hong Kong population was 11/1198 (0.9%).
This parallel IgG EIA system can simultaneously detect HEV antibodies and differentiate between individuals with HEV-A exposure and HEV-C1 exposure and is a valuable tool for investigating epidemiology and risk factors for HEV-C1 infection. The authors look forward to using this assay for wider seroprevalence studies and discovering risk factors for HEV-C1 infection.
This study was published in JHEP Reports (2023 May 15;5(9):100793). Mr. Jianwen Situ serves as the first author, and Dr. Sridhar as the senior author.
Read the full article: DOI: 10.1016/j.jhepr.2023.100793