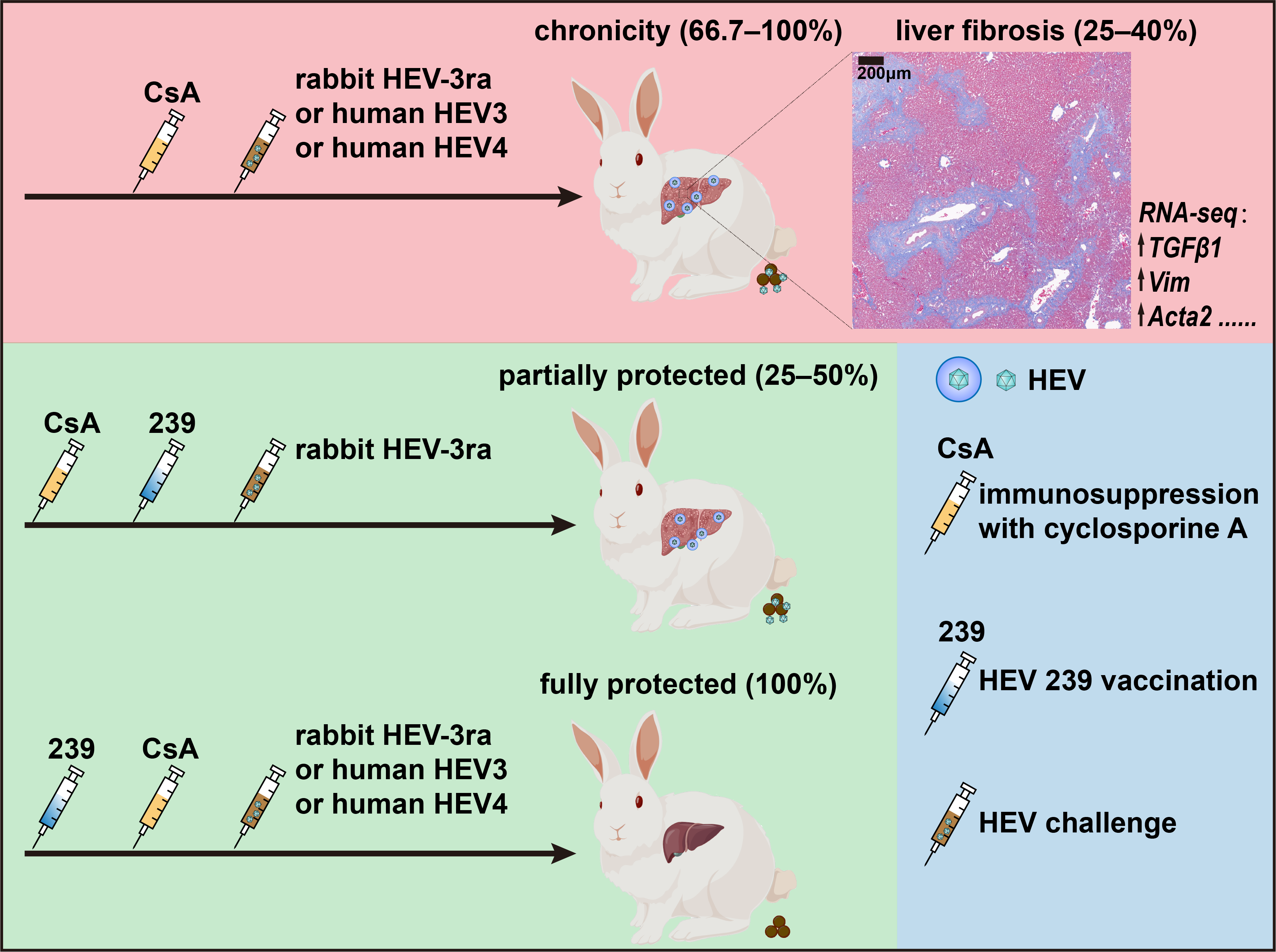
Hepatitis E virus (HEV) has become the emerging cause of viral hepatitis in humans worldwide with genotype 1-4 (HEV1-4) contributing to most cases in humans. Although most HEV infections are acute and self-limiting, chronic HEV infection (defined as persistent infection for >3 months) frequently occurs in immunocompromised individuals, such as organ transplant recipients, and can rapidly result in liver fibrosis and even fatal cirrhosis. The majority of the chronic cases are caused by HEV3, while those caused by HEV4 have recently been described in China. Current treatment options for chronic hepatitis E are limited to reducing the dose of immunosuppressants or using the broad-spectrum antiviral agent ribavirin. The lack of a specific anti-HEV drug for treating chronic hepatitis E necessitates a prevention strategy for this high-risk group. HEV 239 vaccine can effectively protect the general population from HEV1 and HEV4 infection. However, the vaccine efficacy in immunocompromised individuals remains unknown.
Given the burden and the unmet medical need for chronic hepatitis E, the researchers from Peking University recently established a cyclosporine A-induced immunocompromised rabbit model of both chronic HEV3 and HEV4 infection. In this study, immunocompromised rabbits challenged with rabbit-derived HEV3 (HEV-3ra) are an ideal model for recapitulating chronic HEV infection as seen in organ transplant patients, which captures key features of viral kinetics, HEV-host interactions, chronic disease progression including rapid liver fibrosis, and extrahepatic replication. Of note, although human-derived HEV3 and HEV4 are less infectious in rabbits, chronic infection as defined by viral fecal shedding was also established in two-thirds of immunocompromised rabbits, which might facilitate the study of both genotype 3 and 4 chronic infection as well as the study of HEV cross-species transmission. This model was further validated for the evaluation of anti-HEV candidates.
Interestingly, the vaccine efficacy was also assessed by using this model. Vaccination completed before immunosuppression conferred full protection against both HEV3 and HEV4 infections, but vaccination during immunosuppression was only partially protective and the efficacy did not improve with increased or additional vaccine doses. This result provide an preliminary preclinical data for future study of the optimal vaccination strategy in immunocompromised patients.
In summary, this immunocompromised rabbit model, together with the previously established porcine model (by Prof. Meng) and humanized mice models, are valuable for understanding the pathogenesis of chronic hepatitis E, as well as for evaluating antiviral agents and vaccines. This study was conducted by Dr. Qiyu He, Dr. Lin Wang, Prof. Fengmin Lu, Prof. Ling Wang and their colleagues from Peking university with the contributions from Dr. Qiuwei Abdullah Pan from Erasmus MC–University Medical Center, Rotterdam, the Netherlands and Dr. Xing Liu and Prof. Xin Yin from Harbin Veterinary Research Institute, Chinese Academy of Agricultural Sciences, China.
The study was published in Hepatology in March, 2022. Publication link: https://aasldpubs.onlinelibrary.wiley.com/doi/10.1002/hep.32455