From hepatitis E virus to rat hepatitis E virus (ratHEV), hepeviruses clearly require a One Health approach to tackle. ratHEV is increasingly recognized as a cause of human hepatitis. Although only a few human cases have been reported in Hong Kong, Spain, France, Canada and recently in mainland China, this low incidence likely reflects diagnostic limitations rather than true absence of the virus. The global circulation of ratHEV in rodent populations, coupled with substantial genetic diversity and inconsistent molecular diagnostic methods, suggests that human infections may be underdiagnosed. Consequently, the clinical impact of ratHEV infection and its role as an aetiological agent of acute hepatitis might be poorly understood.
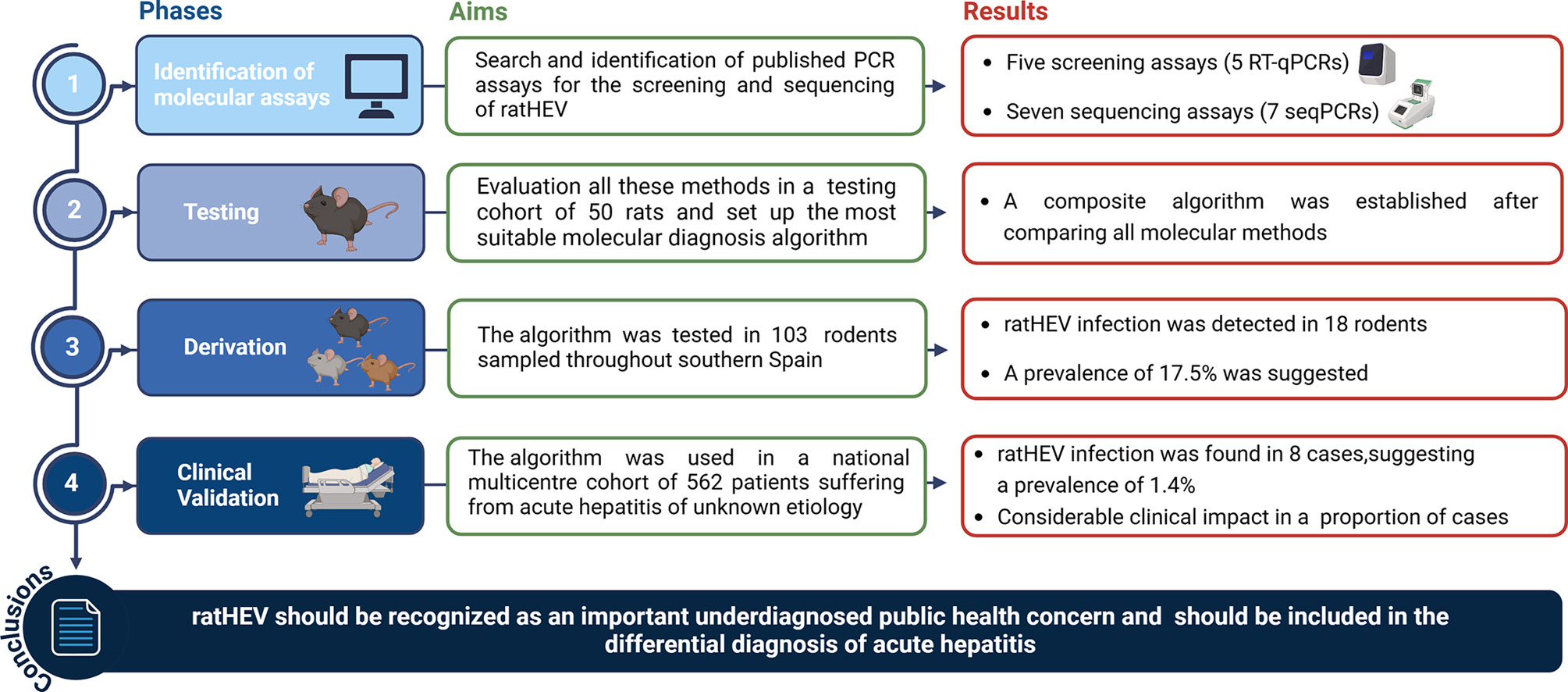
A recent study led by Javier Caballero Gómez, Antonio Rivero and Antonio Rivero Juárez, aimed to determine the frequency of ratHEV as an aetiological agent of acute hepatitis and its clinical impact after the development and optimization of the most suitable molecular diagnostic algorithm. For that, they designed a One Health study in four phases:
- Identification of Molecular Assays – A comprehensive review of existing qPCR and sequencing (seqPCR) primers was conducted to evaluate their efficacy in detecting ratHEV RNA.
- Testing Phase – Various qPCR and seqPCR assays were assessed using samples from 50 Norway rats collected in Córdoba, Spain, an area with documented human cases of ratHEV.
- Derivation Phase – The optimized molecular diagnostic algorithm was applied to a larger rodent cohort (n=103) from four Spanish provinces.
- Clinical Validation Phase – The final algorithm was employed to screen a cohort of 562 patients with acute hepatitis of unknown aetiology from multiple hospitals from Spain.
Remarkably, none of the assays perfectly matched all ratHEV sequences in GenBank and, consistently, considerable differences in the frequency of positive results were detected among the assays evaluated in the testing phase. Eighty percent (40/50) of Norway rats were positive for ratHEV by qPCR, with confirmation via sequencing in 72.5% (29/40). After comparing all available molecular methods, we established a molecular diagnostic algorithm. In the derivation phase, 41 of 103 rodents tested positive by qPCR, with sequencing confirmation in 18 cases (17.5% prevalence). Finally, Of 562 patients with acute hepatitis of unknown aetiology, 51 (9.1%) were qPCR-positive, but only eight (1.4%) were confirmed by sequencing. Infections were detected over three years (2022–2024), predominantly in middle-aged patients (median age: 55 years), with slight male predominance (59.3%). Phylogenetic analysis demonstrated a strong genetic relationship between human and rodent ratHEV strains, supporting zoonotic transmission. Jaundice and fever were significantly more common in ratHEV positive patients than in the overall cohort. One immunocompromised patient presented severe acute hepatitis, acute renal failure, and pancreatitis, demonstrating potential for extrahepatic involvement. Half of the infected patients required hospitalization, and one patient (12.5%) died due to complications.
This study underscores ratHEV as an emerging and underdiagnosed cause of acute hepatitis, necessitating improved molecular diagnostic strategies. The findings suggest that standardized, high-sensitivity qPCR screening (multiplex qPCR-1/qPCR-4) should be incorporated into clinical diagnostics. In this regard, whole-genome sequencing methods, such as NGS, are needed to overcome limitations of current sequencing approaches. One Health surveillance, integrating human and animal monitoring, is critical for understanding transmission dynamics and mitigating zoonotic risk.
Read the full article: Journal of Hepatology 2025 Feb 26:S0168-8278(25)00136-9; DOI: 10.1016/j.jhep.2025.02.027