The safety of the HEV vaccine (Hecolin®) during or shortly before pregnancy remains uncertain, with only two studies from China providing limited insights. These studies had significant limitations such as over 50% elective terminations among pregnant HEV vaccine recipients, which hindered the assessment of natural pregnancy outcomes, and lacked proper documentation on vaccination timing, pregnancy, and outcome detection methods.
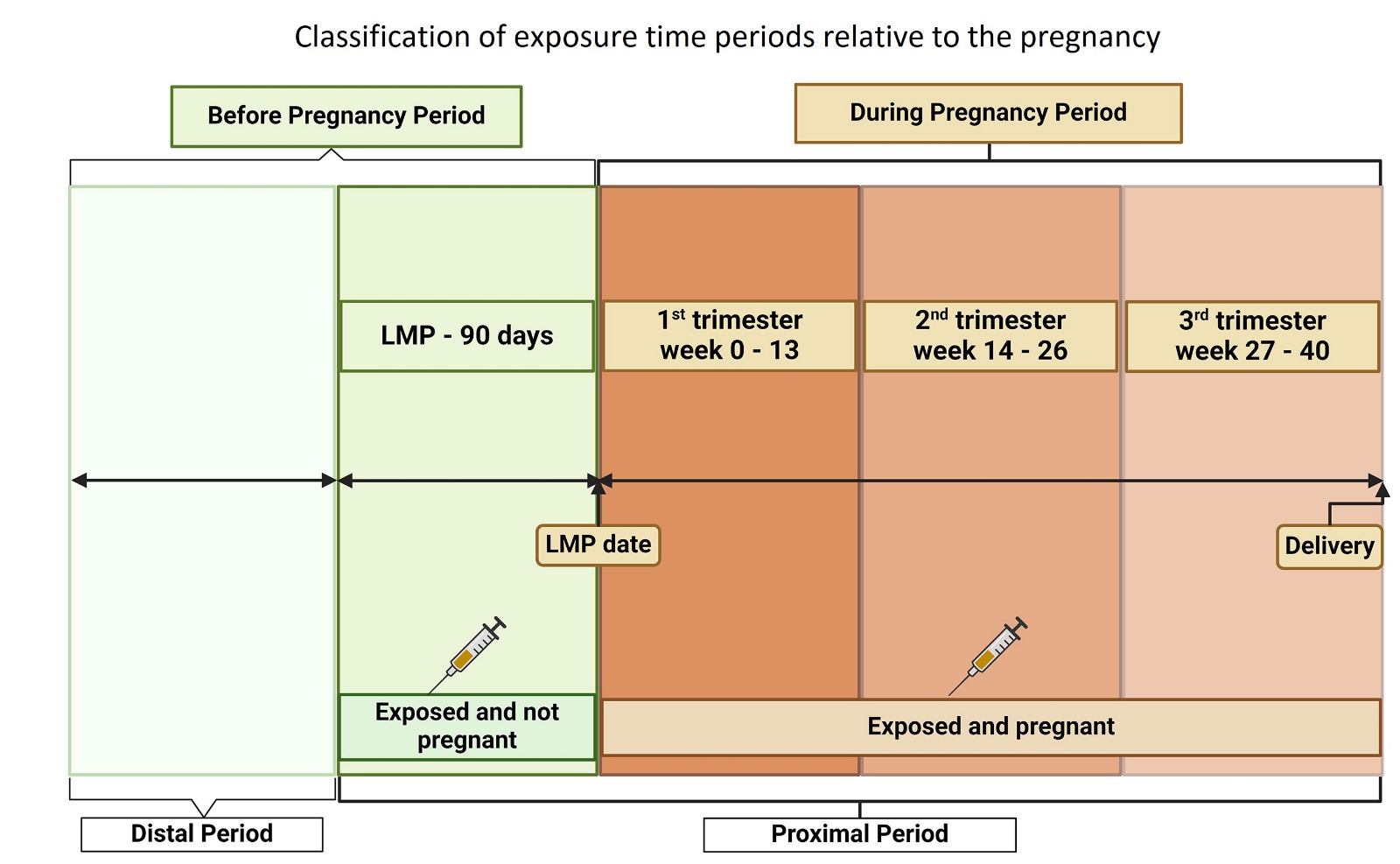
We conducted a large, cluster-randomized trial in 67 villages, focusing on the effectiveness of the HEV vaccine given before pregnancy on HEV infections during subsequent pregnancies up to two years after vaccination. Throughout the trial, we maintained systematic and active follow-up, with frequent direct contact to detect and monitor pregnancies. Women were followed weekly before pregnancy and biweekly after becoming pregnant, with miscarriage diagnoses made by physicians and confirmed by ultrasound. Our outcome completeness was enhanced by using explicit algorithms to analyze data from both trial procedures and a parallel system of routine pregnancy surveillance in Matlab, renowned for its longstanding reproductive surveillance and care system globally.
Our analysis of the main trial data revealed a safety signal for miscarriage, regardless of the timing of doses in relation to pregnancy onset (Zaman K, Julin CH, Aziz AB, et al. Lancet Glob Health. 2024). As responsible investigators, we further examined this relationship to determine if bias could explain the findings. However, we found a two-fold increase in the risk of miscarriage when the HEV vaccine was given shortly before or early in pregnancy. This risk was specific, as no increased risk was observed before 90 days before pregnancy (distal period), during any period for elective terminations, or for stillbirths. The elevated risk remained constant even after various sensitivity analyses.
While the biological mechanism for this effect is unknown, the potential for miscarriage as a side effect of the HEV vaccine has significant public health implications. HEV is a major cause of maternal and perinatal mortality in impoverished regions, particularly South Asia. Vaccination often occurs reactively during unpredictable outbreaks in populations with high illiteracy rates. To avoid the risk of miscarriage, HEV vaccination would need to be avoided during the 90 days before and during pregnancy, a challenging scenario in these settings. Although the relative risk may seem low, the absolute risk, nearly 5% in this study, is not negligible. Policymakers must carefully weigh these risks when considering the use of HEV vaccines during the outbreak.
Read the full article in Lancet Global Health 2024 Aug;12(8):e1300-e1311. DOI: 10.1016/S2214-109X(24)00193-1