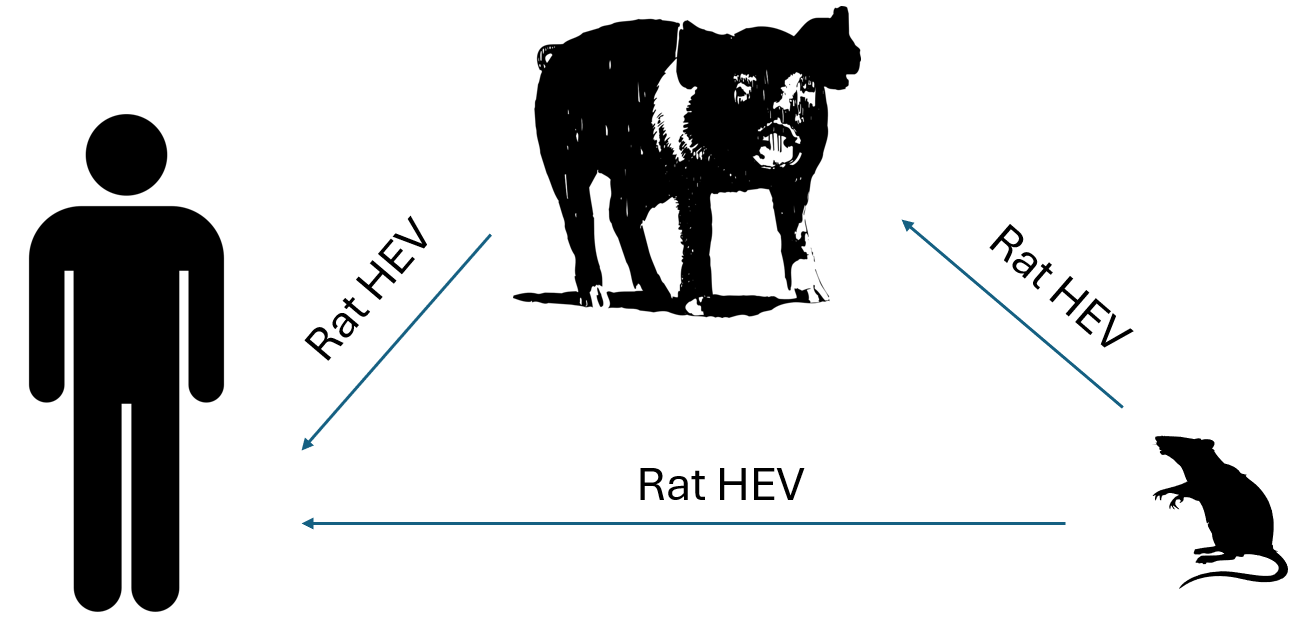
Rat HEV (Rocahepevirus ratti) is an emerging infectious disease with a global concern. Initial experiments in 2013 with rat HEV concluded it to be noninfectious to humans as evidenced by the absence of viral replication in nonhuman primates (NHPs). Infections in humans were not noted until 2018 when it was first reported in an immunosuppressed human case in Hong Kong. Since this initial case, rat HEV has been shown to be infectious to both immunosuppressed and immunocompetent humans, with 21 reported cases. Rat HEV disease has been defined by mild hepatitis but has been demonstrated to progress to persistent infection in immunosuppressed human patients. In general, pigs are a primary source of transmission for human HEV (Paslahepevirus balyani genotypes 3 and 4) strains and could serve a similar role for rat HEV transmission as rats are a common pest found on swine farms worldwide. Rats could transmit rat HEV to pigs which could then be transmitted to humans. Determining susceptibility of pigs to emerging zoonotic rat HEV strains can define potentially new transmission routes to inform public health policy and could provide pathology models for rat HEV disease.
A recent study led by Dr. Kush Kumar Yadav under the direction of Associate Prof. Dr. Scott Kenney from The Ohio State University, United States developed the LCK-3110 infectious clone and explored its infectious ability to known primary reservoirs of human HEV, namely pigs. They reveal that pigs can serve as an intermediate host for rat HEV infection in humans and should be studied further for this transmission potential.
A rat HEV LCK-3110 infectious clone was prepared, and replication was assessed in multiple cell lines including swine testicular (ST), human lung (A549), mouse subcutaneous tissue (LMTK), and human hepatoma cells (huh7 S10-3), hepG2/C3A cells. The researchers aimed to identify the cell line best suited to study rat HEV replication using the capped RNA transcripts. ST cells demonstrated a higher number of ORF2 positive cells as determined using the flow cytometry analysis. Furthermore, they demonstrated that capped RNA transcripts of the rat HEV LCK-3110 strain were infectious when intrahepatically injected into the livers of gnotobiotic pigs. Viral RNA in feces and serum was detected until the study termination date, 35 days post-inoculation (dpi). Rat HEV RNA was also detected in bile, liver, spleen, ileum, kidney, urinary bladder, urine, feces, brain, and cerebrospinal fluid (CSF). To further characterize the pathogenicity of rat HEV LCK-3110 virus in conventional pigs, intestinal contents derived from the intrahepatically inoculated gnotobiotic pigs sacrificed on day 35 were used for the preparation of an infectious stock which was further used in the pathogenicity study. They found that rat HEV produced a higher fecal titer in pigs at week 1 post-inoculation than US-2 (Paslahepevirus balayani genotype 3) inoculated pigs (positive control). US-2 fecal shedding appeared to taper off beginning at 3 weeks post-infection, whereas the rat HEV-infected pigs continued to increase fecal shedding through the end of the study, 35 dpi. Sentinel pigs in the rat HEV-infected room began to shed virus in their feces at week 2 post-comingling and appeared to begin tapering off at week 4 post-comingling. Both rat HEV- and US-2 HEV-inoculated pigs had moderate, multifocal lymphohistiocytic hepatitis at 35 dpi. The liver enzyme tests indicated no significant differences between the groups in alanine aminotransferase (ALT) and aspartate aminotransferase (AST) during the period of infection, suggesting that no serious liver damage was induced in the pigs by rat HEV infection.
They also demonstrated the ability of rat HEV derived from conventional pig feces to replicate in human hepatoma cell lines. Additionally, rat liver cells (clone 9) were also susceptible to rat HEV LCK-3110 strain. Based on the findings, authors concluded that pigs could serve as an intermediate source of emerging zoonotic rat HEV transmission to humans. More surveillance and the development of rat HEV specific serological tools are needed to appropriately address pigs as potential transmission vectors for rat HEV.
Dr. Kush Kumar Yadav is a Postdoctoral Associate at Dr. XJ Meng’s lab, Department of Biomedical Science, College of Veterinary Medicine, Virginia Tech University. He gained 6 years of valuable experience working with HEV during his graduate career (MS and PhD) under the supervision of Dr. Kenney.
Dr. Scott P. Kenney is an Associate Professor, at Center for Food Animal Health, Department of Animal Sciences, Department of Veterinary Preventive Medicine, The Ohio State University.
Read the full article published in PNAS Nexus (2024 Jul 1;3(7):pgae259): Rat hepatitis E virus cross-species infection and transmission in pigs – PubMed (nih.gov)