Hepatitis E virus (HEV) is a leading cause of acute viral hepatitis globally. Currently, no causal treatment or universally licensed vaccine exists. Several recombinant HEV vaccines have been developed, including the commercially available Hecolin® vaccine, primarily targeting HEV genotype 4. However, the crossprotectivity of these vaccines against other HEV genotypes, particularly HEV genotype 3 (HEV-3), remains uncertain.
A recent study led by Dr. Martin Eiden, from Institute of Novel and Emerging Infectious Diseases at the Friedrich-Loeffler-Institut, Federal Research Institute for Animal Health, Germany, assessed the efficiency of Hecolin® against swine derived genotype HEV-3 infection. This evaluation was performed in an established pig infection model with proven exceptionally high susceptibility to HEV-3 (Dähnert et al. 2018). In addition, a new genotype 3 based p239 vaccine variant, p239(gt3), that is homologous to the challenge strain, was designed and evaluated in the study. Pigs were divided into three groups: One group received Hecolin®, another received p239(gt3), and the control group remained unvaccinated. All pigs were subsequently exposed to HEV-3 to assess the vaccines’ effectiveness. While all vaccinated pigs developed high titers of neutralizing antibodies, neither vaccine provided complete protection against HEV-3 infection (Figure 1). In the Hecolin® group, two out of four pigs exhibited virus shedding, and viral RNA was detected in the bile and/or liver of three out of four pigs in both vaccinated groups. Only one pig in each group was fully protected. While Hecolin® partially prevented HEV shedding, the novel p239(gt3) vaccine suppressed virus shedding in all infected pigs. These findings underscore the necessity for further development of HEV vaccines that offer broad protection against multiple genotypes and the importance of using suitable animal infection models.
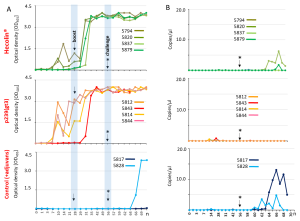
Figure. Antibody development after vaccination starting with initial immunisation, boost at day 28 and challenge at day 56. Figure displays the individual curves of antibody titer obtained from ELISA analysis (A). Results of RT-qPCR of shed HEV RNA from fecal samples over the course of vaccination and challenge (B). Arrow indicates date of boost. Arrow with star indicates date of infection. n: necropsy
The data demonstrate the limited protective efficacy of both vaccines against HEV-3 infection, particularly regarding sterilizing immunity. Hecolin®, the only registered vaccine so far, is available only in China and Pakistan with proven efficiency against HEV-4. Hecolin® was evaluated in numerous studies and demonstrated efficacy against HEV-1 and rabbit HEV strains. However, data on cross-protection and effectiveness against HEV-3 are limited (WHO TRS N°1016). Another factor that has so far been underestimated and given little attention in vaccination studies is the exceptionally high susceptibility of pigs to HEV-3, with as few as 6.5 copies being sufficient to cause infection (Dähnert et al. 2018). Comparable titration studies for rabbits or primates are lacking, making pigs a suitable model for evaluating Hepatitis E vaccine efficacy, especially for potential human applications. Further studies on human and swine-derived HEV-3, as well as HEV-4 strains, are needed to determine the protective efficacy of both established and novel vaccine candidates.
Read the full article (One Health. 2024 Jan 6:18:100674.): DOI: 10.1016/j.onehlt.2023.100674
References:
Dähnert L, Aliabadi E, Fast C, Hrabal I, Schröder C, Behrendt P, Protzer U, Groschup MH, Eiden M. 2024. Immunisation of pigs with recombinant HEV vaccines does not protect from infection with HEV genotype 3. One Health. 2024 Jan 6;18:100674. doi: 10.1016/j.onehlt.2023.100674.
Dähnert L, Eiden M, Schlosser J, Fast C, Schröder C, Lange E, Gröner A, Schäfer W, Groschup MH. 2018. High sensitivity of domestic pigs to intravenous infection with HEV. BMC Vet Res 14(1):381
WHO. WHO Reference number: WHO TRS N°1016. https://www.who.int/publications/m/item/recombinant-hepatitis-e-vaccines-annex-2-trs-1016