Porcine-derived pancreatic enzyme replacement therapy (PERT) is indicated for treating exocrine pancreatic insufficiency due to cystic fibrosis or other conditions in both paediatric and adult patients, and thus the overall size of this patient population is substantial. A recent work by Thornton et al1, from Canada, described three cases of chronic hepatitis E in persons with cystic fibrosis (pwCF) after lung transplantation. Because most pwCF took porcine-derived pancreatic enzyme replacement therapy (PERT), the authors suspected PERT as a plausible source of transmitting hepatitis E virus (HEV) to these patients. Alarmingly, the authors found that 44% of PERT capsules were positive for HEV viral RNA.
These intriguing findings have been highlighted in a Commentary by Frericks et al2. In their opinion, this study should be seen as an encouragement to further investigate HEV seroprevalence in other pwCF cohorts and ultimately raise the awareness of a potential risk of HEV transmission through animal-derived medications in general, and call validation studies from in other CF and transplant centers.
Indeed, this prompted a follow-up study led by Kamar et al3, from Toulouse, France. In their institution, all heart, liver, kidney and pancreas transplant patients are followed in the same department. Lung transplant patients are followed in another department and these patients were not included in this study. For more than 15 years, all solid organ transplant patients presenting with liver enzymes levels abnormalities, neurological symptoms and/or suspected HEV-associated kidney injury are tested for HEV RNA in the serum (and the stools when possible). Between 1 January 2010 and 31 December 2023, 5071 transplant patients were followed in our department, that is, 3694 kidney transplant patients, 889 liver transplant patients, 325 heart transplant patients, 153 simultaneous kidney-pancreas transplant patients and 10 pancreas alone transplant patients. Of these, 99 (1.95%) patients received PERT produced by two different manufacturers during this period. HEV infection was diagnosed in 161 out of the 4972 transplant patients who did not receive PERT (3.2%), and 2 out of the 99 transplant patients (2%) of transplant patients who received PERT. HEV RNA was detected in only 2/59 (3%) of PERT capsules. Their data suggest that the risk of HEV infection is similar in patients given or not PERT. However, they cannot rule out PERT as a source of HEV transmission.
Zhou et al4 emphasized these findings in a broader context (figure). Currently, many types of medicines and vaccines contain pork products. For example, heparin, an injectable anticoagulant indicated for prevention or treatment of thrombotic events, is predominantly of porcine origin. An increase of anti-HEV seroprevalence was found in individuals undergoing heparin therapy in cohort from Portugal, although a causative relation has not yet been proven. Thus, it is time to raise the awareness, but more importantly to take actions by the regulatory bodies and pharmaceutical industries to assess the risks as well as implement measures to prevent HEV transmission from porcine-derived medical products.
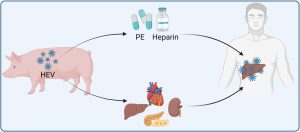
The potential risk of transmitting hepatitis E virus (HEV) to humans through porcine-derived medicinal products such as heparin or pancreatic enzyme (PE), and through xenotransplantation of porcine-derived cells, tissues, or organs. The figure was generated by using BioRender.
Futuristically, xenotransplantation of porcine-derived cells, tissues or organs is approaching clinical reality (Figure). Attributing to the recent advancement of gene editing to generate ‘humanised’ pigs, xenotransplantation of porcine organs including kidney and heart is rapidly emerging in clinical testing. However, one of the major challenges for clinical use of porcine organs is the potential risk of transmitting pathogenic viruses to the human recipients. However, there is currently no laboratories to provide complete screening of pathogens especially zoonotic viruses in the setting of xenotransplantation. Zhou et al4 call the establishment of such technologies and guidelines to prevent zoonotic transmission in xenotransplantation, in particular for viruses like HEV that pigs serve as native reservoirs.
Key references
- Thornton CS, Waddell BJ, Congly SE, et al. Porcine-derived pancreatic enzyme replacement therapy may be linked to chronic hepatitis E virus infection in cystic fibrosis lung transplant recipients. Gut. 2024 Apr 15:gutjnl-2023-330602.
- Nicola Frericks , Volker Kinast, Eike Steinmann. Potential risk of porcine-derived pancreatic enzyme medication for the cross-species transmission of hepatitis E virus. 2024 May 31:gutjnl-2024-332577.
- Nassim Kamar, Olivier Marion, Florence Abravanel, Laure Esposito, Arnaud Del Bello, Jacques Izopet. Porcine-derived pancreatic enzyme replacement therapy: a cause of hepatitis E virus transmission? Gut. 2024 Jul 2:gutjnl-2024-332744.
- Zhou J, Liu K, Pan Q. Porcine-derived pancreatic enzyme replacement therapy linking to chronic hepatitis E: broad implications. Gut. 2024 Jun 14:gutjnl-2024-332975.

