Many gaps remain in the understanding of the Hepatitis E Virus (HEV) lifecycle. Notably, viral factories induced by HEV have not been documented yet and it is currently unknown whether HEV infection leads to cellular membrane modelling as many positive-strand RNA viruses do. HEV genome encodes the ORF1 replicase, the ORF2 capsid protein and the ORF3 protein involved in virion egress. Previously, L. Cocquerel’s laboratory demonstrated that HEV produces several isoforms of the ORF2 protein that perform distinct functions in the HEV lifecycle. Among them, the infectious ORF2 (ORF2i) form is the structural component of infectious particles.
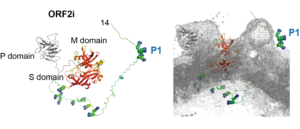
In their recent study, Bentaleb C, Hervouet K, et al. generated monoclonal antibodies that specifically recognize the particle-associated ORF2i form, and antibodies that recognize the different ORF2 isoforms (Bentaleb C, Hervouet K, et al., 2022, Cell Mol Life Sci.). One antibody, named P1H1 and targeting the ORF2i N-terminus, recognized delipidated HEV particles from cell culture and patient sera. Importantly, AlphaFold2 modelling demonstrated that the P1H1 epitope (P1) is exposed on HEV particles.
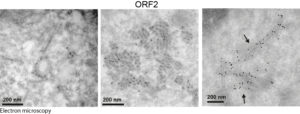
Next, they used their antibodies in confocal and electron microscopy approaches to probe infectious particles and viral factories in HEV-producing cells. They identified an unprecedented HEV-induced membrane network containing tubular and vesicular structures that were enriched in ORF2 and ORF3 viral proteins. Besides, they found that these structures depend on ORF2i capsid protein assembly and ORF3 expression, but not on HEV replication (Bentaleb C, Hervouet K, et al., 2022, Cell Mol Life Sci.). Furthermore, by using the same approaches as in a previous study aiming at characterizing the ORF1 replicase (Metzger K, Bentaleb C, et al., 2022, Front Microbiol.), they showed that the ORF1 protein and genomic RNA were also enriched in the HEV-induced subcellular structures.
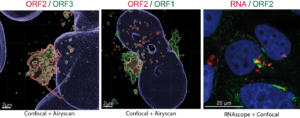
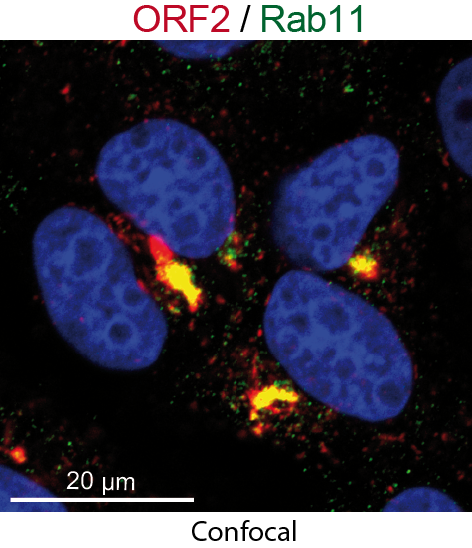
Finally, extensive colocalization analyses of viral proteins with subcellular markers and silencing experiments demonstrated that these structures were derived from the endocytic recycling compartment (ERC) for which Rab11 is a central player (Bentaleb C, Hervouet K, et al., 2022, Cell Mol Life Sci.). Hence, cellular ERC likely serves as a viral factory for HEV. Taken together, this study demonstrated that HEV hijacks the ERC during its lifecycle and forms a membrane network of vesicular and tubular structures that might be the hallmark of HEV infection. This work opens new diagnostic and therapeutic perspectives to fight HEV.
These studies were conducted in the laboratory of Laurence Cocquerel (Univ.Lille/CNRS/Inserm/Pasteur Institute of Lille) and published in Frontiers in Microbiology (April 2022) and Cellular and Molecular Life Sciences (December 2022).
Read the original articles:
DOI: 10.1007/s00018-022-04646-y
https://doi.org/10.3389/fmicb.2022.828636