HEV infection is associated with nerve root and plexus sequelae, such as Guillain-Barré syndrome and neuralgic amyotrophy, in a significant proportion of HEV-infected individuals worldwide. Additionally, HEV-associated central nervous system (CNS) disorders, such as encephalitis, myelitis, cerebral ischemia, and seizures, have also been reported in infected patients. These neurological sequelae are almost exclusively associated with zoonotic genotype 3 HEV infection, and to a lesser extent, zoonotic genotype 4 HEV infection. Although the exact mechanism how HEV infection leads to neurological diseases remains largely unknown, there is evidence suggesting that HEV may cross the blood-brain barrier (BBB) to invade CNS.
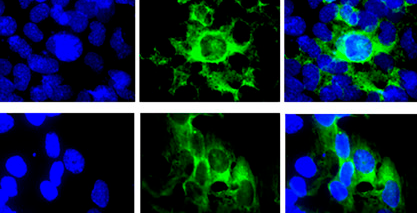
A recent study led by Dr. Debin Tian and Prof. X.J. Meng from the Virginia Polytechnic Institute and State University, USA, delineated a potential mechanism of HEV-associated neuroinvasion. They first conducted an in vitro study using a blood-brain barrier (BBB) cell culture model and found that both quasi-enveloped and non-enveloped HEV particles can cross the BBB in vitro in a tumor necrosis factor-alpha (TNF-α) independent manner without significant disruption of tight junction structure of BBB. Both quasi-enveloped and non-enveloped forms of HEV particles can productively infect cell lines of human brain microvascular endothelial cell and astrocyte which are important components of BBB.
The team subsequently conducted an in vivo study by intravenously inoculating specific-pathogen-free pigs with both quasi-enveloped and non-enveloped genotype 3 human HEV (US2 strain), respectively. HEV was detected in the CNS tissues of a proportion of infected pigs. The pigs that were tested positive for HEV in CNS developed histological lesions in brain and spinal cord, and also had higher levels of proinflammatory cytokines TNF-α and interleukin-18 (IL-18), when compared to the inoculated pigs that are HEV-negative in CNS. Based on the findings in their study, they proposed a potential mechanism of HEV neuroinvasion via direct infection of brain microvascular endothelial cells of BBB.
This study was published in Proc. Natl. Acad. Sci. USA. 2022;119 (24):e2201862119. Read the full research article https://doi.org/10.1073/pnas.2201862119.